Playbook
About 37 million Americans have diabetes, and one-quarter of this population uses insulin to manage their condition. [1] Given high demand, the insulin marketplace should be more competitive. But high costs still cause some people with diabetes to ration or skip their prescribed doses. [2] While progress is occurring, the persistence of high prices shows how the market for drug treatments can incentivize profits over affordability and access for patients.
This playbook traces the commercial and regulatory factors that influence the cost of insulin.
What is "Patent Thicketing"?
Insulin has been around for a hundred years, but the first generic competitors to some of the most common insulin products didn’t make it to market until recently. One reason is “patent thicketing,” in which manufacturers make incremental changes to their products and seek additional patents to extend exclusivity. For example, to prolong patents on Humalog and NovoLog, the manufacturer made changes to the injectable pen used to deliver insulin. Click the “Show the Patents” button to extend patent protection by making a patented change to the injectable pen.
See how drug makers use patents to encourage people with diabetes to use their band of insulin:
From Production to Patient
After years of persistent high prices, regulatory action is needed. Incentives introduced by manufacturers and a market with a limited number of competitors contribute to historically high insulin prices. However, new developments prompted by public concern [8,9] are having an impact. The market may be at a tipping point in favor of increased affordability.
Learn about the key factors in production and marketing that influence a drug's price:
Market Share
Concentrated Market, Rising Prices
Despite patient demand, the market is highly concentrated, with 90% controlled by three manufacturers: Eli Lilly (product: Humalog), Novo Nordisk (NovoLog), and Sanofi (Lantus).[10] Historically, prices and average rebates have consistently risen for these types of insulin. A recent Congressional probe found Humalog and NovoLog prices have increased 1219% and 627% respectively since their launch dates in 1996 and 2000. By comparison, the price of Humira, a different biologic, has risen 471% over that same time period.[11]
Competition
More Affordable Biosimilar Insulin Slow to Enter the Market
In 2020, insulin was reclassified legally as a biologic, which streamlined the pathway for generic, or biosimilar, versions. [12 13 14]The introduction of biosimilars typically brings down prices.[15] The first interchangeable insulin biosimilar, Semglee, was approved by the FDA in 2021.[16] Semglee is a long-acting insulin. Humalog and NovoLog, which are fast-acting, have yet to see FDA-approved biosimilar competitors in the U.S. The introduction of biosimilars is not always an easy journey. Incumbent manufacturers have strong incentives to maintain their market share and often engage in activities to extend their exclusivity beyond the date of their original patent.
Patenting
Patent “Thicketing” Extends Protections for Name-Brands
A key strategy by drug companies to slow the introduction of biosimilar competitors is patent “thicketing,” where manufacturers make incremental changes to seek additional patents on legacy products. While some reformulations may improve the safety or efficacy of a drug, others may have less robust impact and operate mainly to extend patent exclusivity. Additionally, for products delivered by a medical device, such as an injectable pen common among insulin products, the combination of the medication and the device patents opens the door for even longer patent protection. For example, while patents have expired on certain formulations of Humalog and NovoLog, the devices used to deliver those medications still have active patents.[17]
Regulation
New Law Limits Cost to $35 for Medicare Patients
The Inflation Reduction Act of 2022 includes provisions aimed at lowering prescription drug costs and improving access for Medicare beneficiaries.[18] It limits monthly cost-sharing for insulin products to no more than $35 per month, which represents a substantial savings for those eligible for Medicare – a decline under Medicare Part D to no more than $420 a year from as much as $2,333.[19]
Regulation
Insulin Makers Respond to $35 Cap with Price Cuts
Some Americans not on Medicare got an unexpected benefit[20] when the three major insulin manufacturers also reduced prices on select products.[21] Eli Lilly, maker of Humalog, matched the IRA’s cap for insulin products distributed through participating pharmacies for patients with commercial insurance and those uninsured.[22] Novo Nordisk announced it will lower list prices by up to 75% for several products as of 2024.[23] And Sanofi announced a $35 monthly cap on Lantus for people with private insurance beginning in 2024.[24] While the exact reasons for these proactive price decreases are not clear, one possibility is an effort to maintain market share ahead of the introduction of biosimilars. In March 2022, CivicaRX, a non-profit generic drug and pharmaceutical company, announced plans to bring to market a generic insulin interchangeable with Lantus, Humalog, and NovoLog at a price of no more than $30 per vial.[25]
Regulation
States Also Move to Control Insulin Costs
There is also significant activity at the state level to control insulin costs. In 2019, Colorado became the first state to implement a price cap on out-of-pocket costs at $100. Since then, nearly two dozen more states have passed similar measures. These protections, however, are typically limited to insurance products over which the state has explicit control, including those sold on the Affordable Care Act Health Insurance Marketplace and those covering state employees.[26]
Patient Usage
Many Diabetes Patients Ration Insulin Dosages
When individuals with diabetes are unable to afford insulin, they ration it. A recent study found that 1.3 million insulin users were forced to ration their insulin at least once in 2021. Insulin rationing includes instances in which a diabetes patient skips a dose altogether, takes less than prescribed, or delays refilling their prescription. Cost is the principal reason for missed doses.
Distribution
Pharmacy Benefit Managers Play a Significant Role in Pricing
While much of the focus on insulin’s high cost has been directed at manufacturers, the ultimate price encountered by consumers is also impacted by insurance plans, pharmacy benefit managers (PBMs), pharmacies, and wholesalers. One study determined that while the manufacturer list price of 32 insulin products increased 40.1% between 2014 and 2018, the net price decreased by 30.8% on average.[27] The difference is accounted for by discounts negotiated between various intermediaries within the supply chain. Health plans saw a 24.7% reduction in insulin expenditures. However, the share of expenditures retained by PBMs increased by 154.6%, the share retained by pharmacies increased 228.8%, and the share retained by wholesalers increased 74.7%, all over the same time.[28]
Distribution
Understanding Who Benefits from Discounts
The recent announcements from Eli Lilly and Novo Nordisk of their intentions to lower the list prices of their insulins will likely have a significant impact on the manufacturer-PBM rebate dynamic. Over ten years of gross-to-net data show that both Humalog and NovoLog have been subject to ever-increasing discounts, and as of 2022 Q1, both Eli Lilly and Novo Nordisk were returning approximately 85% of Humalog and NovoLog list prices back to PBMs in the form of rebates. Per their announcements, Eli Lilly and Novo Nordisk over time will reduce the list prices of Humalog (by 70%) and NovoLog (by 75%), effectively transferring a substantial proportion of the discount from PBMs directly to patients, since patients’ cost sharing obligations are typically tied to drugs’ list prices.
Distribution
Direct-to-Consumer Discounts a New Model for the Industry?
The price shifts from Eli Lilly and NovoNordisk are good news for patients. While it’s tempting to think this could be a model across the industry, it’s possible this is only sustainable in isolated cases where there is high political and cultural pressure to push experimentation. However, those tracking the industry closely will be paying attention to the ripple effects of this announcement, not just on other manufacturers of insulin, but across drug classes.
Distribution
The FTC Warns Companies About Using Rebates
PBMs also play an important role in determining the list of generic and brand-name drugs covered by a specific health plan. There is evidence that exclusionary rebates for PBMs may have contributed to maintaining high prices for insulin. Exclusionary rebates occur when a manufacturing company incentivizes the PBM to exclude certain medications from their formularies, or list of approved drugs. This is often done to maintain market share for their brand-name products over available generics.
Distribution
Number of Excluded Drugs Balloons
Over the last ten years, the number of drugs eliminated from PBM availability lists has grown significantly, from zero to 600 drugs excluded from formularies across the three biggest PBMs. The U.S. Federal Trade Commission has warned companies that engage in this behavior, indicating it would be a violation of federal law. The FTC singles out insulin as a prominent example of a drug whose price has been impacted by high rebate fees to PBMs and other intermediaries.
Imagining the Patient Experience
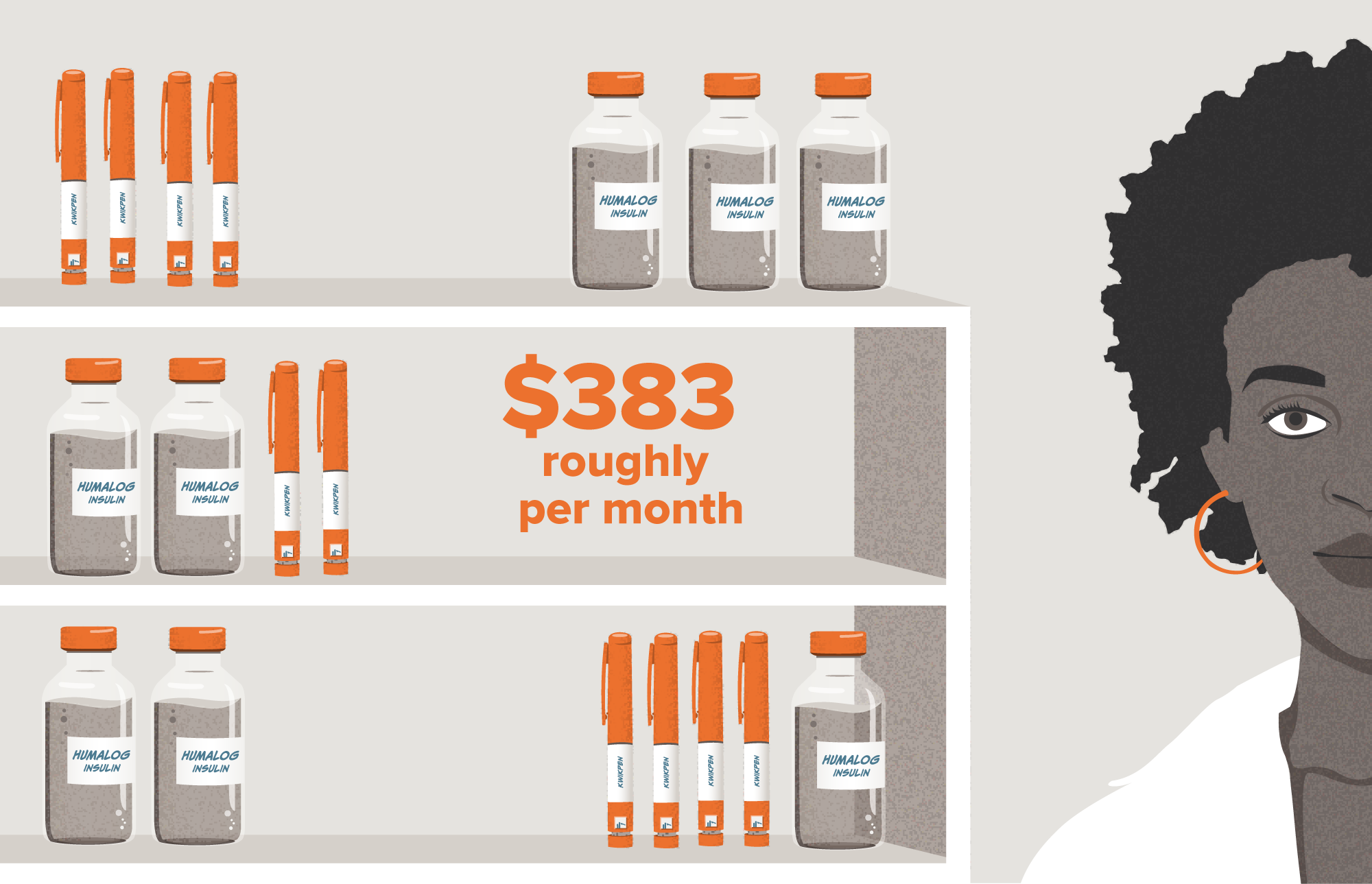
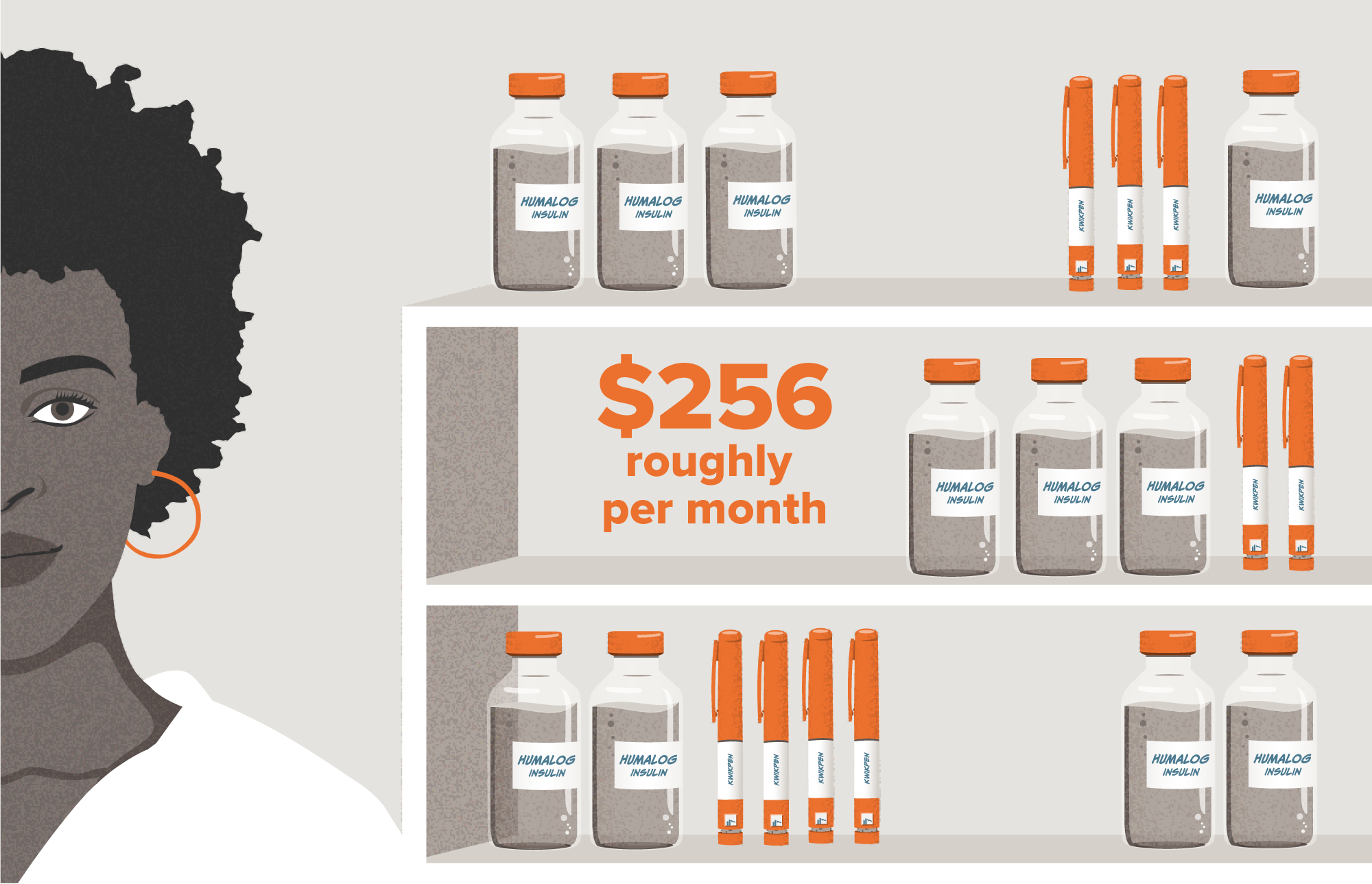
Changes in the insulin market and planned price reductions may have a profound impact on costs for some patients. For example, people treating diabetes with Eli Lilly’s Humalog could see annual savings of $1,500.
See how a commercial insurance (high-deductible health plan) patient’s insulin costs could change in Q4 2023.
Note: Patient scenarios are meant to be illustrative only. Prices are based on publicly available information when possible and good-faith estimates when prices were not available.
Government Reforms Lead the Way to Lower Insulin Prices
Insulin products have come under intense cost scrutiny, culminating in significant reforms directly applicable to the Medicare population. Reforms and price reductions are beginning to expand into the commercial market.
Profit Incentive Still Powerful
While reforms gain steam, the market for this high demand treatment shows how major price increases, patent thicketing, and complex negotiations with pharmacy benefit managers can incentivize profits over affordability and access.
Price Caps are Effective
The government capping the consumer price of insulin in Medicare has been shown to be an effective tool in lowering the cost of this drug, as well as having a profound impact across the marketplace improving patient access and lifting barriers to affordability. Policy makers should consider taking similar measures to improve access and removing barriers for other crucial medications.